Important Research Findings by Professor Liu Jinping's Team Published in Nature Communications
Recently, the research team led by Professor Liu Jinping from School of Chemistry, Chemical Engineering, and Life Sciences has achieved a significant breakthrough in sodium-ion battery research. Their study entitled Understanding Pillar Chemistry in Potassium-containing Polyanionic Materials for Long-lasting Sodium-ion Batteries has been published in Nature Communications. The primary institutional affiliation is Wuhan University of Technology, with doctoral candidates Liu Wenyi, Cui Wenjun, and Yi Chengjun as co-first authors. Professor Liu Jinping, and Associate Professor Wang Zhuo from Zhengzhou University are corresponding authors. Notable collaborators include Researcher Sang Xihan, also from Wuhan University of Technology.
Sodium-ion batteries (SIBs), characterized by abundant resources and low cost, demonstrate tremendous potential for large-scale energy storage and electric/hybrid vehicle applications. Compared with lithium-ion batteries, SIBs exhibit superior advantages in fast-charging capability and wide-temperature operation. Although hard carbon anodes offer high capacity in typical SIBs, they suffer from unavoidable issues like sodium dendrite formation, which severely compromises battery safety and cycle life. Potassium-containing polyanionic compounds, characterized by spacious ion transport channels and stable open-framework structures, offer a promising alternative as safe and cost-effective anode materials for SIBs. However, their sodium storage behaviors have been rarely studied and the mechanism remains unclear.
Notably, the sodium storage mechanism in potassium-containing compounds proves more complex than their sodium-containing counterparts. Beyond elucidating structure and phase transitions, a critical yet unresolved question persists: Does Na+substitution for K+occur through ion exchange during cycling? For this material family, fundamental controversies remain regarding the existence, mechanism, and extent of K+/Na+ion exchange. Deciphering the working mechanism of potassium-containing polyanionic materials is crucial for understanding long-term cycling stability and guiding rational material design.
To this end, Professor Liu Jinping's team developed an interference-free KTiOPO4thin-film electrode model system. By integrating in-situ/operando spectroscopy, spherical aberration-corrected electron microscopy, and density functional theory calculations, they comprehensively unveiled the Na+storage mechanism. For the first time, both experimentally and theoretically, they demonstrated the incomplete K+/Na+exchange, the pillaring/stabilizing effect of 0.15 residual K+on the lattice tunnel structure, and the "near-zero strain" behavior (only 3.9%) of Na+intercalation and deintercalation in the material. They achieved long-cycle stability of over 10,000 cycles and excellent fast-charging safety (no sodium deposition at a 5C rate). At the same time, the prepared powder electrode exhibited a quite high capacity and was able to work efficiently at a commercial-grade areal capacity of 2.47 mAh cm−2. The assembled quasi-solid-state sodium-ion pouch cells still had high safety and long-cycle stability under extreme abuse conditions.
This study introduces the novel concept of “pillar chemistry”, thereby expanding the paradigms of conventional intercalation chemistry. It not only quantifies the degree of ion exchange, clarifies the sodium storage mechanism, and but also explores the low-cost, long-cycle, and high-safety sodium ion cathode materials. In addition, the proposed “pillar chemistry” strategy opens new avenues for designing polyanionic electrode materials incorporating large hetero-ions.
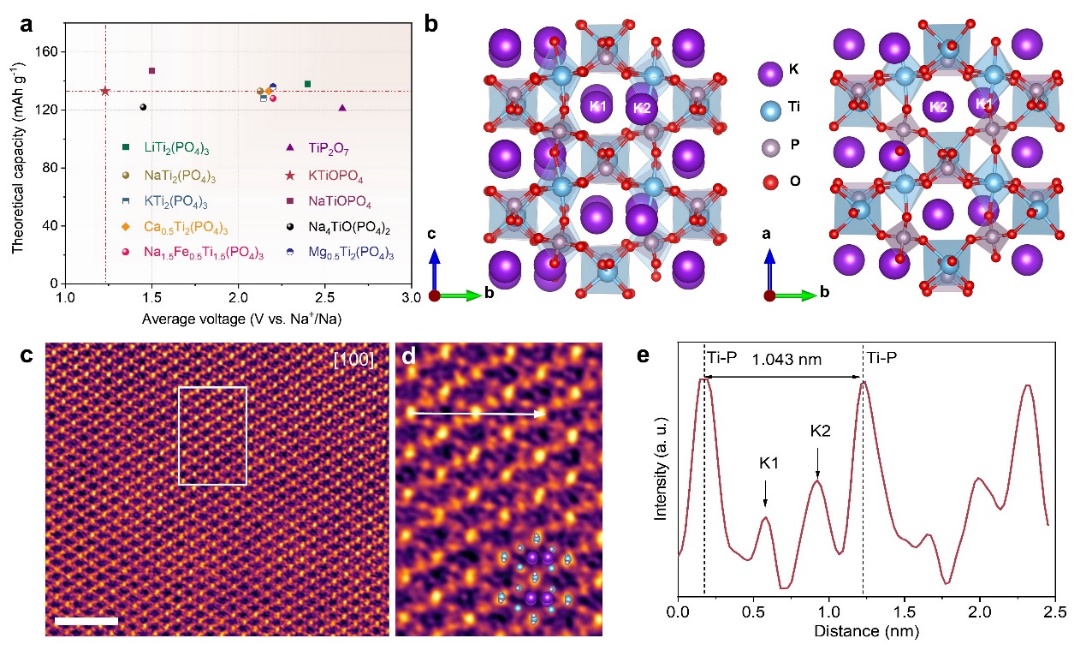
Fig. 1. Advantage and Structural Characterization of KTIOPO4Cathode Material
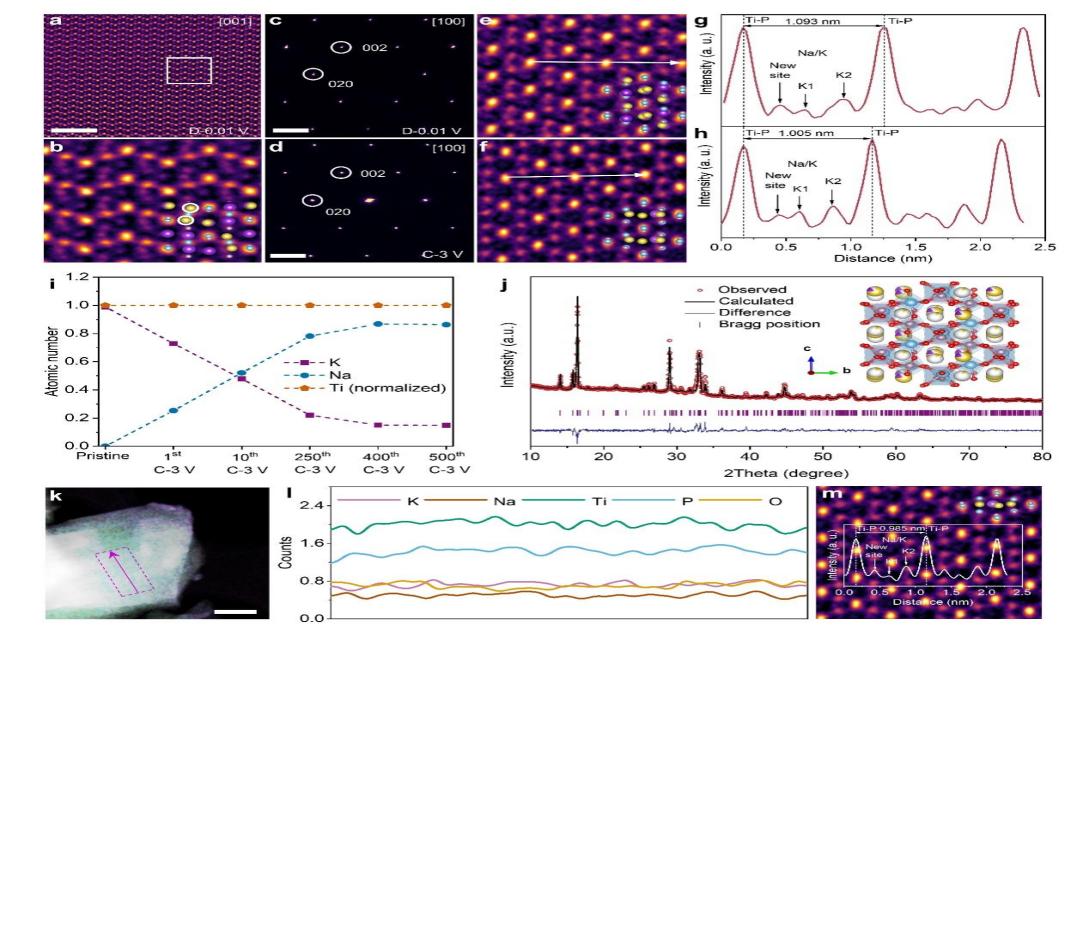
Fig. 2. Atomic Structure Evolution and Na+/K+Ion Exchange Mechanism Analysis of KTIOPO4Cathode during Cycle
Article links: https://www.nature.com/articles/s41467-024-54317-8
Contributor: Liu Jinping Reviewer: Li Junsheng
