Professor Sun Taolei's Team Publishes Latest Findings on Nanomaterial-Regulated Amyloid Self-Assembly in CEJ
Recently, the research team led by Professor Sun Taolei from the Hubei Provincial Key Laboratory of Nanomedicine for Neurodegenerative Diseases of Wuhan University of Technology published their latest research findings in Chemical Engineering Journal (IF: 13.3, a top journal in the first tier of the Chinese Academy of Sciences' journal classification). They proposed a design criteria for nano-biological interfaces based on non-covalent interactions, which provides theoretical and technical support for the development of nano-drugs that can inhibit the abnormal aggregation of amyloid proteins, such as the Aβ peptide in Alzheimer's disease. Wuhan University of Technology is listed as the sole corresponding institutional affiliation. Prof. Gao Guanbin, Prof. Shen Lei, and Prof. Sun Taolei are the co-corresponding authors, and Master's student Mu Qingxue is the first author of the paper.
Neurodegenerative diseases, such as Alzheimer's disease, are closely associated with the abnormal folding and fibrillation of amyloid proteins, such as the Aβ peptide. Most existing research focuses on the function of artificial nanomaterials in mimicking the role of "molecular chaperones" and stabilizing protein homeostasis through non-covalent interactions. However, unified design criteria remain elusive. Using the composite structure of zero-dimensional nanoparticles and two-dimensional nanosheets (NPs@NSs) as a model, this study systematically analyzed the impact of the nano-biological interfacial energy effects on the behavior of amyloid proteins. By precisely regulating the interface energy of nanocomposites, the molecular mechanism underlying its influence of interfacial energy regulation on amyloid self-assembly and fiber disassembly was revealed for the first time, and the universality of relevant design criteria was verified.
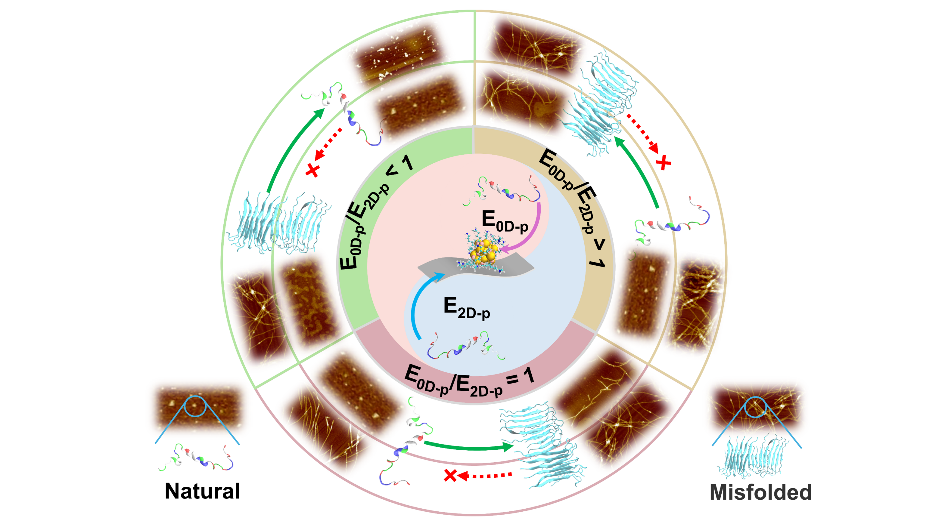
Schematic Diagram of Design Criteria for Nano-biological Interface Based on Non-covalent Interactions
Through molecular dynamics simulations and experimental verifications, the research team proposed the core indicator of the "non-covalent interface affinity energy ratio (E0D-p/E2D-p)". When the affinity energy ratio of 0D nanoparticles to 2D nanosheets for the Aβ peptide is lower than 1 (e.g., for AuNCs@rGO and AuNCs@BP), the composites can stabilize the natural conformation of Aβ monomers and disrupt the β-sheet structure of mature fibers through dual-site synergy. When the ratio is close to or higher than 1 (e.g., for AuNCs@Ti3C2Tx), the inhibitory effect of the material is significantly diminished.
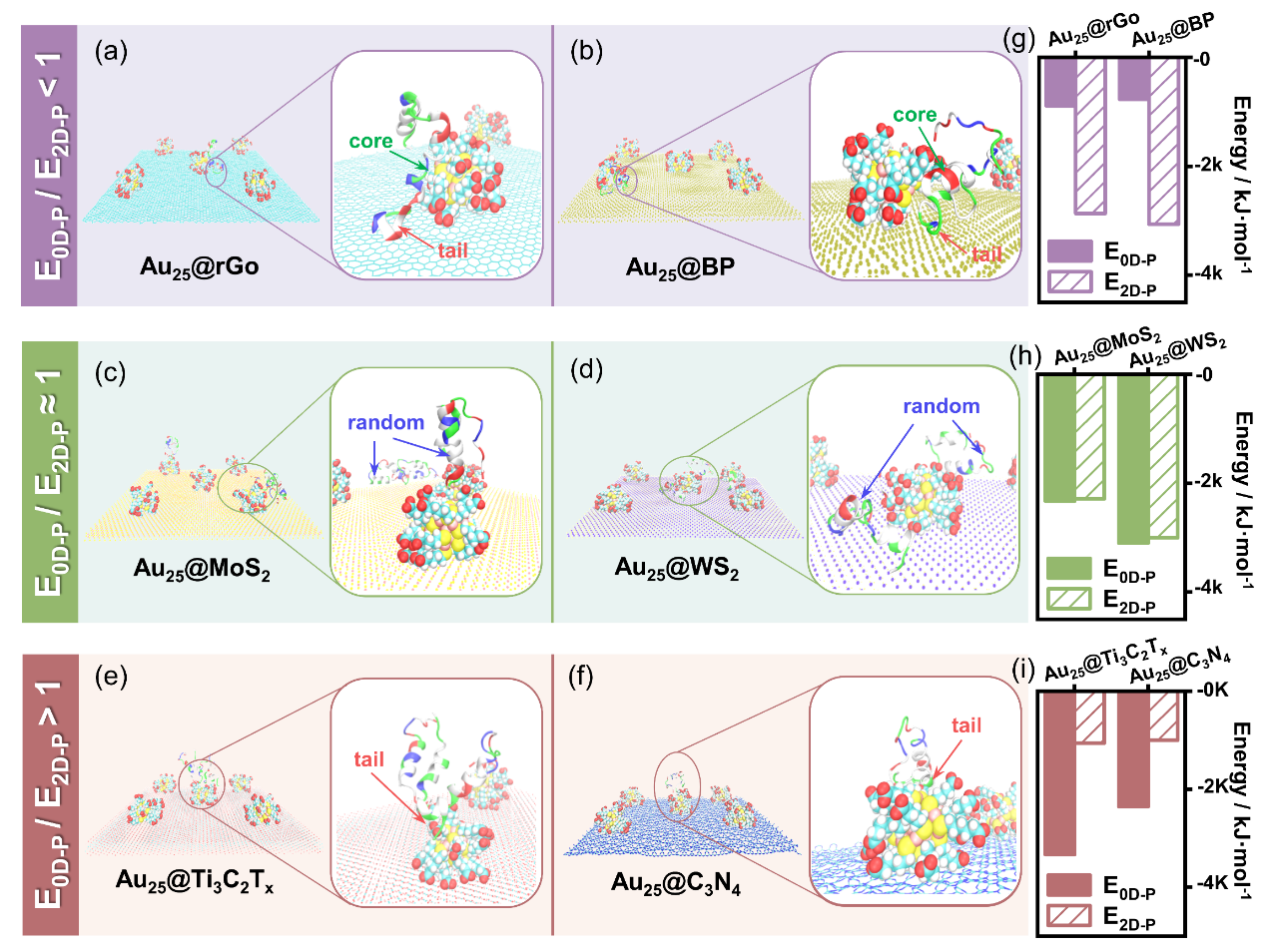
Effects of NPs@NSs with Different Non-covalent Interface Affinity Energy Ratios (E0D-p/E2D-p) on the Behavior of Amyloid Protein
The research team experimentally verified the above-mentioned criteria. On one hand, from the perspective of inhibiting the fibrosis of protein monomers, the Aβ40 monomers which were co-incubated with AuNCs@rGO still maintained their natural conformation after 72 hours, with the degree of fibrosis decreasing by 92%. On the other hand, in terms of disassembling mature fibers, under the same conditions, AuNCs@rGO could reduce the β-sheet content of mature fibers from 81% to 50%. Moreover, the novel material AuNCs@BP exhibited a stronger disassembly ability. More importantly, at a concentration of 100 mg/L, AuNCs@BP was non-toxic to nerve cells (PC 12) and human astrocytes (HA), and significantly enhanced the survival rate of Aβ-damaged cells.
Review experts commented that this study established, for the first time, the quantitative relationship between the interfacial energy of nanocomposites and the regulatory function of amyloid proteins. Gao Guanbin, the corresponding author, stated that "this criterion is applicable not only to Alzheimer's disease but also can be extended to the development of nano-drugs for other amyloid-related diseases, such as Parkinson's disease. This achievement has been validated by various in vitro models, and the next step will be to explore their pharmacokinetics and therapeutic efficacy in animal models. "
This research was supported by the National Natural Science Foundation of China (Grant Nos. 52273110 and 52372271), the National Special Support Program for High-Level Talents, and the Hubei Provincial Top Young Talents Program. This research provides a novel paradigm for the precise design of anti-amyloid nanodrugs, marking a significant step forward for nanomedicine in the treatment of neurodegenerative diseases. With the progress of subsequent clinical translation research, this discovery may offer hope to tens of millions of Alzheimer's disease patients worldwide.
For detailed data, please refer to Chemical Engineering Journal, Vol. 507 (2025), with the article number 160577.
Article information: Mu Qingxue, Gao Guanbin*, Zhang Zijun, Zhang Bin, Gu Zhenhua,
Liu Xinglin, Yu Liangchong, Shen Lei*, Sun Taolei*. A validated
criterion for engineering 0D@2D nanocomposite to modulate amyloid
peptide self-assembly and fibril disassembly[J]. Chemical Engineering
Journal, 2025, 507, 160577.
Article links:https://www.sciencedirect.com/science/article/pii/S1385894725013981
